[COVID19]COVID Arm, Delayed Large Local Reactions to mRNA vaccine
페이지 정보
작성자 작성일21-08-30 10:25 조회3,187회 댓글0건짧은 주소
-
 짧은주소 :
https://www.suwonma.com/b/hs0201/1640
주소복사
짧은주소 :
https://www.suwonma.com/b/hs0201/1640
주소복사
관련링크
본문
[COVID19]COVID Arm, Delayed Large Local Reactions to mRNA vaccine
- Delayed Large Local Reactions to mRNA-1273 Vaccine against SARS-CoV-2 (N Engl J Med 2021; 384:1273-1277)
▶What Is COVID Arm?
Soreness and swelling at the injection site are common reactions to the Moderna and Pfizer-BioNTech COVID-19 vaccines. Sometimes, these common symptoms are followed by a less-common itchy rash and other symptoms. This later reaction is known as COVID arm.
COVID arm can be uncomfortable, but it’s rare, and harmless. It typically occurs around 1 week after vaccination from the first or second shot.
COVID arm is mostly associated with the Moderna vaccine.
In this article, we’ll clue you in on the symptoms and treatments of COVID arm. We’ll also explain why it happens.
▶Symptoms
COVID arm is a harmless immune system reaction that some people have after getting the Moderna vaccine. A 2021 case reportTrusted Source showed that the Pfizer-BioNTech vaccine may also cause COVID arm but appears less likely to do so. The Johnson & Johnson vaccine has not been associated at all with this side effect.
COVID arm is a delayed hypersensitivity skin reaction that occurs on or around the injection site. Its symptoms show up several days to 1 week or more after the first or second vaccination.
One 2021 case studyTrusted Source of people with this condition found that COVID arm symptoms appeared 7 days after the first shot, and 2 days after the second.
Symptoms of COVID arm include:
- itching, which can be intense
- a red or discolored rash that varies in size from quarter-sized to very large in some instances, the rash may spread to your hands or fingers
- swelling
- pain
- skin feels warm to the touch
- hard lump under your skin where the injection took place
If you’re hearing about COVID arm a lot, you may be concerned that you will have this reaction. Keep in mind that COVID arm is relatively rare and never dangerous. Millions of people around the world are getting vaccinated, so even small occurrences of vaccine reactions and side effects are getting lots of attention.
▶Why does this happen?
COVID arm is thought to be an immune system reaction. Your immune cells are responding to the muscle cells which have absorbed the mRNA vaccine. The vaccine produces the SARS-CoV-2 spike protein, which the immune system thinks is an infection that needs to be fought. This is referred to as an overexuberant immune response.
Since the COVID-19 vaccines are new, we don’t know definitively what exact mechanism triggers COVID arm symptoms. This reaction and others are continuing to be studied worldwide.
▶How long does it last?
The symptoms of COVID arm typically last from 3 to 5 days. COVID arm won’t escalate to a life threatening condition or serious allergic reaction. It is not associated with anaphylaxis.
Usually, the symptoms of COVID arm resolve on their own. However, if your symptoms are severe or if you feel very uncomfortable or worried, talk with a medical professional. They may be able to recommend medications, such as prednisone, that can help your symptoms resolve quickly.
▶Can you treat it?
Treating COVID arm will not reduce your immune system’s response to the vaccine. Your immune system has already indicated it’s responding robustly.
COVID arm should also not stop you from getting your second shot. In some instances, your doctor or vaccination provider may recommend you alternate arms if you had a strong skin reaction to your first vaccine.
Even though it’s not serious, COVID arm can be uncomfortable. At-home treatments that reduce pain, swelling, and itching include:
- cool compresses
- topical steroids
- topical pain medication
- oral antihistamines
- acetaminophen or nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen
▶Why you should still get a vaccine
COVID arm is a minor annoyance which will disappear within a few days. COVID-19 is a life threatening condition, and the vaccine is highly protective against the virus SARS-CoV-2, which causes COVID-19.
If you’re worried about COVID arm, keep in mind that the protective benefits of the COVID-19 vaccine far outweigh the risks associated with COVID arm or developing COVID-19 itself. It’s also important to talk with your doctor to learn more about the COVID-19 vaccine.
▶The bottom line
COVID arm is a delayed hypersensitivity reaction that occurs mostly from the Moderna vaccine. Onset is typically around 1 week after vaccination. Itching, pain, and swelling are the main symptoms.
COVID arm is a relatively rare occurrence, which should not be confused with the early onset arm reactions caused by many vaccines. It resolves on its own within a few days. You may want to consider talking with your doctor to learn more about the COVID-19 vaccine’s benefits and side effects.
▶N Engl J Med 2021; 384:1273-1277
Delayed Large Local Reactions to mRNA-1273 Vaccine against SARS-CoV-2
TO THE EDITOR:
Baden et al.1 report on a phase 3 clinical trial of the mRNA-1273 vaccine against SARS-CoV-2, and they provide information on immediate injection-site reactions, which were observed in 84.2% of the participants after the first dose. The trial also showed that delayed injection-site reactions (defined in that trial as those with an onset on or after day 8) occurred in 244 of the 30,420 participants (0.8%) after the first dose and in 68 participants (0.2%) after the second dose. These reactions included erythema, induration, and tenderness. The reactions typically resolved over the following 4 to 5 days. However, these reactions were not further characterized, and links between reactions after the first dose and those after the second dose were not provided to inform clinical care. (Fig 1)
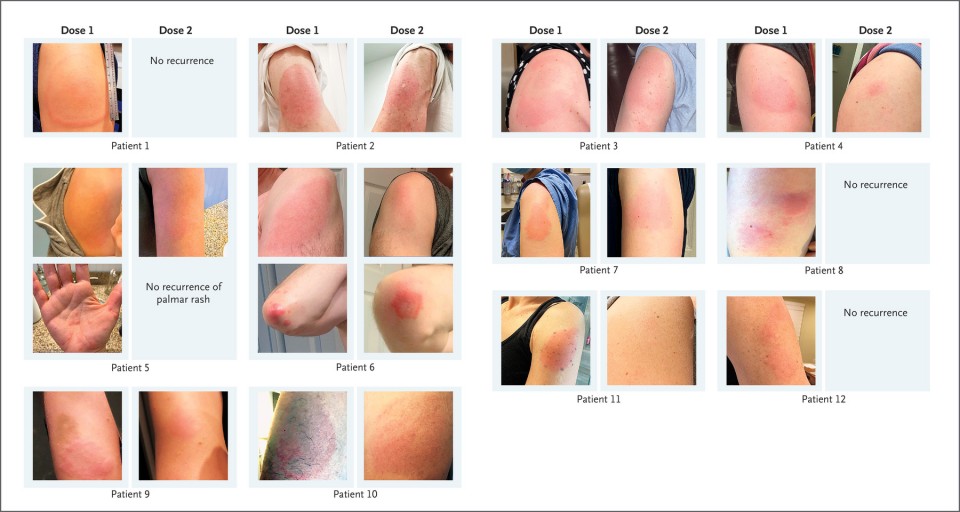
Patients with Remarkable, Delayed, Large Local Reactions to the mRNA-1273 Vaccine.
We have also observed delayed large local reactions to the mRNA-1273 vaccine, with a median onset on day 8 (range, 4 to 11) after the first dose. These reactions had a variable appearance (Figure 1). Here, we report on a series of 12 patients with these reactions, all of which appeared near the injection site after complete resolution of the initial local and systemic symptoms associated with vaccination. Five of the reactions were grade 3 plaques (≥10 cm in diameter) (Table 1). Some patients had concurrent systemic adverse effects, and among these patients, 2 had additional skin findings. Most patients received treatment for their symptoms (e.g., with ice and antihistamines). Some patients received glucocorticoids (topical, oral, or both), and 1 patient received antibiotic therapy for presumptive cellulitis. The symptoms resolved a median of 6 days after onset (range, 2 to 11).
Our suspicion of delayed-type or T-cell–mediated hypersensitivity was supported by skin-biopsy specimens obtained from a patient with a delayed large local reaction who was not among the 12 patients described here. Those specimens showed superficial perivascular and perifollicular lymphocytic infiltrates with rare eosinophils and scattered mast cells (see Fig. S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org).
Given that neither local injection-site reactions nor delayed-type hypersensitivity reactions are contraindications to subsequent vaccination,2 all 12 patients were encouraged to receive the second dose and completed their mRNA-1273 vaccination course. Although half the patients did not have a recurrence of large local reactions, three patients had recurrent reactions that were similar to those after the initial dose, and three patients had recurrent reactions that were of a lower grade than those after the initial dose. The median onset of cutaneous symptoms after the second dose (day 2; range, 1 to 3) was earlier than that after the first dose (Table 1).
Clinicians may not be prepared to address delayed local reactions to the mRNA-1273 vaccine. Given the scale-up of mass vaccination campaigns across the world, these reactions are likely to generate concerns among patients and requests for evaluation. These reactions have not been consistently recognized, guidance regarding the second dose of vaccine has varied, and many patients have unnecessarily received antibiotic agents. We hope this letter encourages additional reporting and communication regarding the epidemiologic characteristics, causes, and implications of these delayed cutaneous reactions, since this information might allay the concerns of patients, encourage completion of vaccination, and minimize the unnecessary use of antibiotic agents.
댓글목록
등록된 댓글이 없습니다.